The information below provides basic information to enhance the readers’ understanding of cardiac electrophysiology and drug-induced arrhythmia.
We’ve attempted to remove all jargon whenever possible. Definitions of key terms have been added to improve readability.
Brief overview of Cardiac Electrophysiology
The mechanical process of heart contraction is a result of an electrical stimulus in the muscle tissue. Normally, a specialized region of heart cells within the sinoatrial (SA) node is the origin of the electrical impulse and determines the frequency of heart contraction. Thus, the SA node is considered the heart's pacemaker.
Electrical stimulation (called depolarization) is a result of regulated ion balance within heart cells. At rest, sodium (Na+) and calcium ion (Ca2+) concentrations are much higher in the environment surrounding heart cells whereas potassium ion (K+) concentration is higher inside the cells. Upon depolarization, specialized gates (called ion channels) in heart cell membranes open, allowing for the rapid influx of Na+ and Ca2+ ions making the interior of the cell more positive. The influx of Ca2+ ions causes activation of the muscle contractile machinery resulting in cell contraction. As a result of the change in cellular charge, K+ ion channels open allowing for the flow of K+ out of the cell. This outward flow of positive ions causes the interior of the cell to become more negative (repolarization) and resets the cycle.
One important component of heart K+ ion channels is encoded by the the human Ether-à-go-go-Related Gene (hERG). This gene encodes for a protein that forms the major portion of the K+ ion channel. hERG is sensitive to drug binding by numerous therapeutic agents which can result in decreased channel function. The interaction of drugs with hERG results in reduced outward flow of K+ ions and delayed repolarization which can cause irregular heart rhythms. Despite this dangerous interaction, the exact reason why drugs bind to this protein is not completely understood.
Drug-induced arrhythmia
Partial list of QT prolonging drugs from CredibleMeds.org
Arrhythmias related to the use of quinidine were first described in the 1920s. Initially, the cause of arrhythmia was not understood, but in the 1960s, a drug-related form of heart arrhythmia was identified (called polymorphic ventricular tachycardia). Quinidine-associated fainting and arrhythmia occurring during treatment, later called Torsade de Pointes by the French physician François Desertenne, was then described on the basis of the characteristic undulating pattern of waves on an electrocardiogram. Arrhythmias related to non-cardiac medications, including antihistamines and antipsychotics, were also reported in the 1960s and 1970s, but they were also poorly understood by clinicians. In the ensuing decades, the mechanism of these arrhythmias, namely prolongation or heart repolarization leading to Torsade de Pointes, was elucidated, and this is now a major focus of drug development and regulatory oversight.
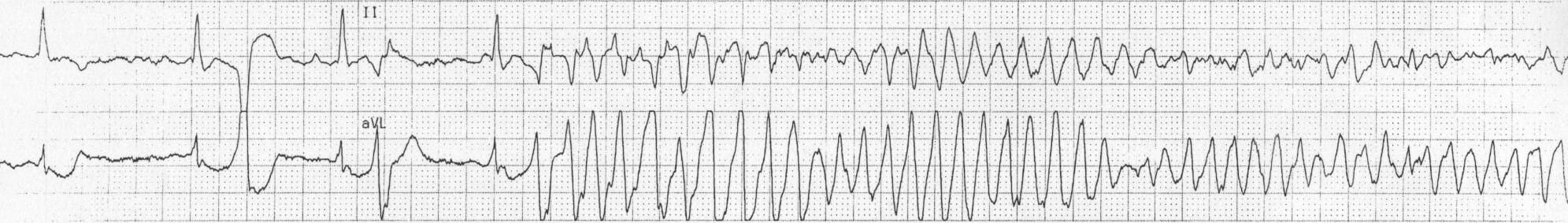
Torsade de Pointes
Torsade de Pointes (TdP) is a French phrase meaning “Twisting of the Peaks” and refers to the nature of the changing electrical complexes seen on an electrocardiogram (below). TdP is a specific type of tachycardic ventricular arrhythmia which can be fatal. While TdP is usually self-correcting, persistence of the event without medical intervention can degenerate into ventricular fibrillation, leading to sudden death. The fleeting nature of TdP events makes it difficult to document during clinical and pre-clinical drug trials. This, coupled with the potential danger of drug-induced TdP, led to the development of guidelines from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use in 2005.
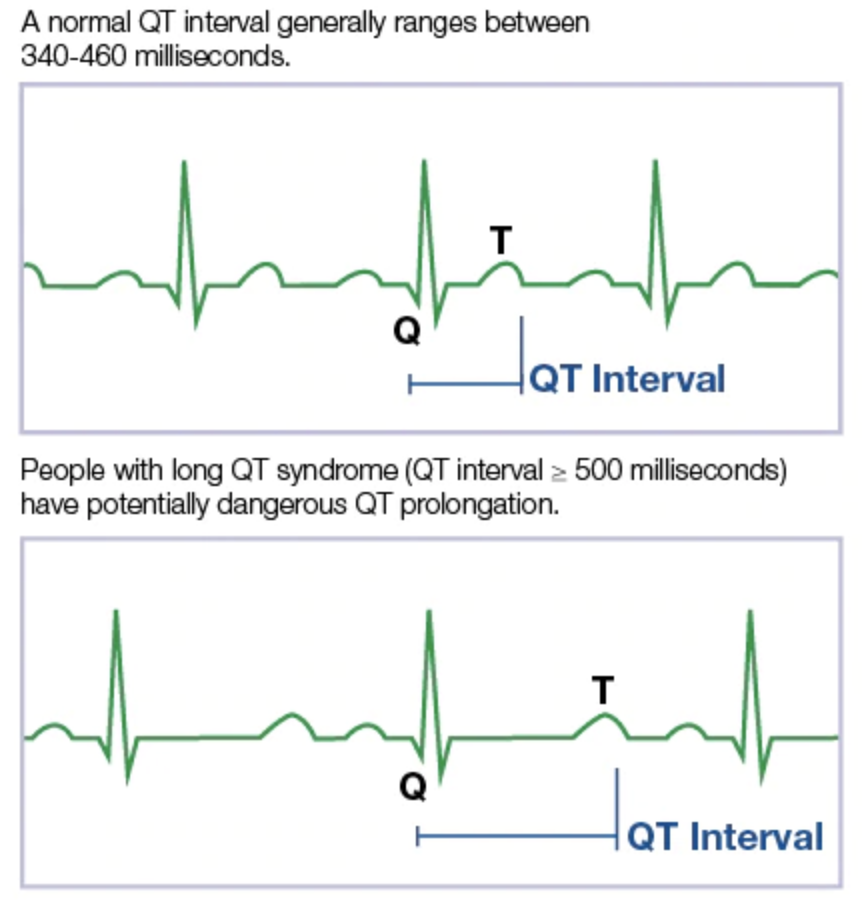
What is QT prolongation and why does it matter?
From MayoClinic.org
TdP has been correlated with QT prolongation: a delay in heart repolarization. Such a delay in repolarization may lead to electrical irregularities which may lead to TdP. The rate-corrected QT interval (QTc) is used as a biomarker of a drug’s effects on heart repolarization and was chosen due to its high sensitivity for predicting TdP. In some instances, the development of potential drugs may be discontinued early in the process if it demonstrates any significant likelihood of prolonging the QTc in order to prevent lethal outcomes in humans.